FAQ
Ques No.1. What is Haemovigilance?
Haemovigilance is a set of surveillance procedures covering the whole transfusion chain from the collection of blood and its components to the follow-up of its recipients intended to collect and assess information on unexpected or undesirable effects resulting from the therapeutic use of labile blood products and to prevent their occurrence and recurrence. It is an important tool for improving safe blood transfusion practices in a country.
Ques No.2. What is Haemovigilance Programme of India (HvPI)?
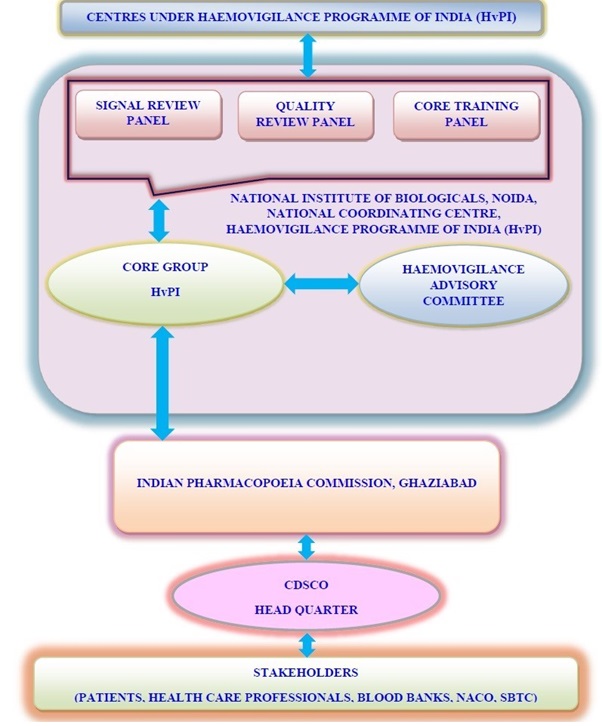
Haemovigilance Programme of India at the national level was launched on 10th December 2012 by National Institute of Biologicals (NIB), NOIDA, Ministry of Health & Family Welfare, Government of India as the National Coordinating Centre (NCC) in collaboration with Indian Pharmacopoeia Commission (IPC), Ghaziabad, Ministry of Health & Family Welfare, Government of India in 90 medical institutions within the country to track Adverse Reactions associated with Blood Transfusion and Blood Product Administration. The activities of this programme are coordinated by a core group, Haemovigilance Advisory Committee, quality, training and signal review panels. The data will be collated and analysed to identify trends, recommend best practices and interventions required to improve patient care and safety. These recommendations will be forwarded to Drugs Controller General (India), Central Drugs Standard Control Organization to formulate safety related regulatory decisions on Blood and Blood Products Transfusion that will be communicated to various stake holder
Ques No.3. What is the objective of HvPI?
Objectives - Haemovigilance Programme of India: o Monitor Transfusion Reactions o Create awareness amongst health care professionals o Generate evidence based recommendations o Advise CDSCO for safety related regulatory decisions o Communicate findings to all key stakeholders o Create National & International Linkages
Ques No.4. What is an Adverse Event?
An adverse event is an undesirable and unintended occurrence before, during or after transfusion of blood or blood component which may be related to the administration of the blood or component. It may be the result of an error or an incident and it may or not result in a reaction in a recipient.
Ques No.5. What is an Adverse Reaction?
An adverse reaction is an undesirable response or effect in a patient temporally associated with the administration of blood or blood component. It may, but need not, be the result of an incident.
Ques No.6. What is Transfusion Reaction Form (TRRF)?
TRRF is a prescribed format used by the centres enrolled under HvPI for reporting adverse transfusion reaction which can be downloaded from the website …………………. & nib.gov.in
Ques No.7. Can we report mild type of reactions not mentioned in the list of nature of reaction in TRRF?
Yes. Mild type of reactions can be reported by using "Other Reactions" tab under Section ….. of Nature of Adverse Reactions.
Ques No.8. What are the mandatory fields in ATR reporting?
The following are the mandatory fields in ATR reporting: " Patient information " Component details " Adverse transfusion reaction details " Outcomes of ATR " Causality Assessment
Ques No.9. Causality assessment done by whom?
Causality Assessment shall be performed by Hospital Transfusion Committee in coordination with the attending Physician of the respective Medical Ward.
Ques No.10. What happens to the submitted data (TRRF)?
The TRR Form submitted to National Coordinating Center -HvPI, is assessed by HvPI Personnel for completeness and correctness. Once the data is assessed, Core Group forward it to the Quality Review Panel for the quality check. Then the data is further forwarded to the Signal Review Panel for the statistical analysis and also for the detection of "Signal". Quality Review Panel and Signal Review Panel in turn provide their recommendations to the Core Group which are further presented to the Haemovigilance Advisory Committee. The recommendations from the Haemovigilance Advisory Committee is forwarded by the Core Group of HvPI to the Indian Pharmacopoeia Commission (IPC), Ghaziabad. Indian Pharmacopoeia Commission (IPC), Ghaziabad then forward the same to CDSCO, Headquarter and CDSCO further takes regulatory decisions and forward them to the Stakeholders (Patients, Healthcare Professionals, Blood Banks, NACO, SBTC etc.).
Ques No.11. How to get enrolled under HvPI?
Medical Colleges/ Institutes/ Hospitals/ Blood Banks of India willing to participate can get enrolled under HvPI for that Head / Incharge of Transfusion Medicine Department / Blood Bank provides the necessary details to the NCC-HvPI by sending the duly filled Enrolment Form either at address - National Institute of Biologicals, Ministry of Health & Family Welfare, Plot No. A-32, Sector-62, Institutional Area, NOIDA - 201 309 (U.P.) or via E-mail at haemovigilance@nib.gov.in NCC verifies the details provided by the Center. After verification, NCC issues the User Id and Password to the Head / Incharge of Transfusion Medicine Department / Blood Bank to access the Haemo - Vigil Software for onward transmission of Transfusion Reactions Reports to NCC.
Ques No.12. Whether NCC- NIB provide any financial support to centres enrolled under HvPI?
Financial support will be given in form of logistic and manpower to the centres enrolled under HvPI by NCC- NIB.
Ques No.13. Will NCC- NIB reveal any information submitted by enrolled centres in this programme under RTI?
The identities of the patient, reporter and institution are never revealed to third party as per our Confidentiality Clause.
Ques No.14. Whether any legal action can be taken against reporting of ATR?
There will be no legal action taken against reporting of ATR since, HvPI is non-punitive programme so, reporters are free from fear of retaliation against themselves or punishment of others as a result of reporting of ATR to NCC- NIB.
Ques No.15. What are reportable under HvPI?
All reactions related to transfusion (serious or non-serious) are reportable under HvPI. Further retrospective data related to blood transfusion reactions can also be reported.