Haemovigilance
Blood transfusion is a lifesaving medical intervention. However, transfusion of blood and blood products may be associated with adverse effects such as transmission of blood borne pathogens including viruses, bacteria and parasites. Non-infectious hazards of transfusion can also harm the patients. Systematic and proactive surveillance of events during and after administration of blood products (Haemovigilance) helps to detect and identify adverse effects of blood transfusion and can be very effective in improving blood quality and safety.
Transfusion of blood and blood products is not without risks and it can lead to complications. Haemovigilance is a continuous process of data collection and analysis of Blood Transfusion related Adverse Reactions in order to investigate their causes and outcomes, and prevent their occurrence or recurrence.
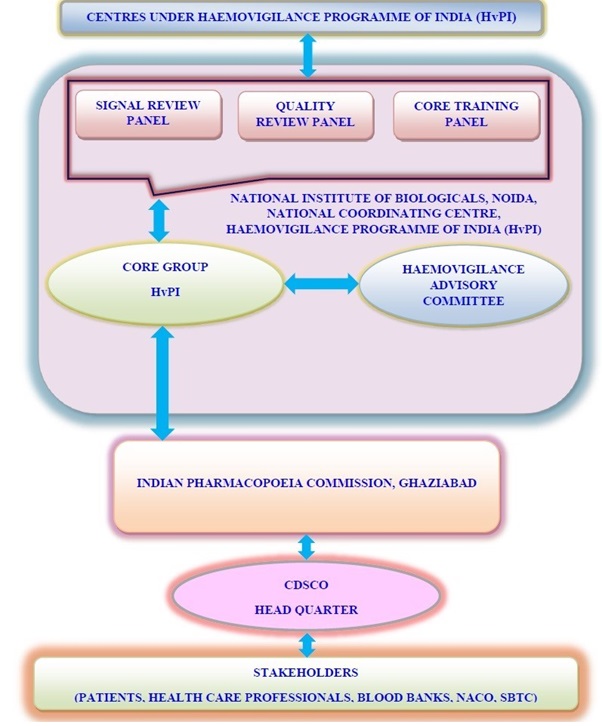
It includes the identification, reporting, investigation and analysis of Adverse Reactions and Events in recipients and blood donors as well as incidents in manufacturing processes, eventually errors and "near-misses". A Haemovigilance system is also an integral part of quality management in a blood system, triggering corrective and preventive actions for the continual improvement of the quality and safety of blood products and the transfusion process.
Importance:
- Provides objective data on transfusion risks
- Increases awareness of transfusion and its complications among hospital staff
- Annual Haemovigilance reports - opportunities for education on transfusion risks
- Hospital Transfusion Committees (HTC's) can use data to review and improve processes involved in handling and administration of blood and components
- Identify potential hazards which may be present but unrecognized